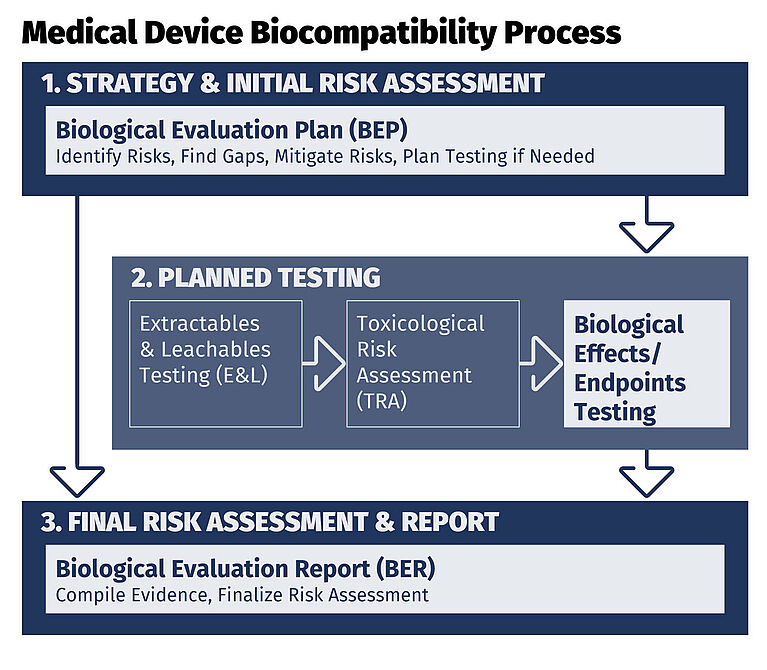
Medical Device Biocompatibility Process
Biological safety evaluation is conducted using a structured, evidence-based approach:
- A Biological Evaluation Plan (BEP) identifies biological hazards, applicable biological effects/endpoints, existing data and data gaps
- If gaps are identified, risk-based biological testing is performed to address potential harms
- Depending on materials and exposure, this may include:
- Extractables and leachables (E&L) studies
- Toxicological risk assessment (TRA)
- All data and rationale are compiled into a Biological Evaluation Report (BER), which documents:
- Biological effects/endpoints evaluated
- Risk acceptability
- Overall biological safety and regulatory compliance